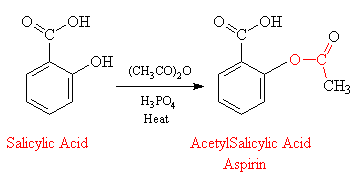
Acetylation of Salicylic Acid
Some P syringae strains make coronatine a jasmonic acid mimic that suppresses salicylic-acid-mediated defence to biotrophic pathogens 49 and induces stomatal opening helping pathogenic bacteria. Aspirin primarily inhibits COX-1 whereas salicylic acid has more balanced COX-1COX-2 activity.

File Acetylation Of Salicylic Acid Mechanism Png Wikipedia
Blocking the platelet cyclooxygenase by acetylation it inhibits thromboxane A2 synthesis a physiological activating substance released by the platelets and which would play a role in the complications of the atheromatosic lesions.

. 170 Salinity stress results in a significant accumulation of ROS in plant roots. Phenol reacts with sodium hydroxide to give sodium phenoxide which then reacts with carbon dioxide in acidic medium to give 2-hydroxybenzoic acid Salicylic acid. Blocking the platelet cyclooxygenase by acetylation it inhibits thromboxane A2 synthesis a physiological activating substance released by the platelets and which would play a role in the complications of the atheromatosic lesions.
Priming with 80 μM salicylic acid SA SA significantly increased germination rate germination potential and germination index of the seeds under 200. Salicylic acid Aspirin is produced and administered via different routes in various doses and forms Arif and Aggarwal 2021. Salts and esters of butyric acid are.
A clue to how ABA production in roots may contribute to the salt stress response is provided by the fact that ABA interacts with H 2 O 2 in plant systemic responses to stresses. The acetylation process instigates alterations at the level of macromolecules and accordingly adjusts the. The flora in the intestines can cause drugs to undergo reactions such as reduction hydrolysis acetylation dealkylation deCO2 and formation of nitrosamine and sulfuric acid conjugates.
Aspirin exerts its antithrombotic effect by irreversibly inhibiting platelet cyclooxygenase through acetylation of serine at position 529 leading to steric hindrance of substrates for the enzymes. Aspirin is the prototype analgesic antipyretic and anti-inflammatory drug. The sodium salt of salicylic acid is.
Acetylsalicylic acid inhibits the platelet activation. βούτῡρον meaning butter also known under the systematic name butanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH 3 CH 2 CH 2 CO 2 H. For example acetylation of aniline gives acetanilide first step in the following equation which undergoes nitration at low temperature yielding the para-nitro product in high yield.
Salicylic acid and its metabolites are predominantly. The modifying acetyl group can then be removed by acid-catalyzed hydrolysis last step to yield para-nitroaniline. Butyric acid ˈ b j uː t ɪ r ɪ k.
Soaking with 05 mM salicylic acid or 01 mM H 2 O 2 at 25C in the dark. The chemical synthesis of acetylsalicylic acid and its role in the unique mechanism of action of aspirin. Aspirin is the oldest and still the most widely used antiplatelet agent.
Salicylic acid is acetylated to form aspirin. Salicylic acid and H 2 O 2 enhanced the germination percentage from 71 to 86 and 87 respectively. Salicylic acid is subsequently predominantly converted into glycine and glucuronic acid conjugates.
The risk or severity of adverse effects can be increased when Mesalazine is combined with Salicylic acid. The natural substitute for synthetic salicylic acid for skin and scalp Neosalyl is a 100 natural and pure salicylic acid for skin and scalp that is organic compared to its synthetic alternative. In organic chemistry acetylation is an organic esterification reaction with acetic acid.
The metabolites of some drugs enter the intestines with bile and are converted into prototype drugs under the action of the flora and then reabsorbed which prolongs the action. Rosenmund reduction is a reaction where acid chlorides are converted into aldehydes by employing hydrogen gas over palladium poisoned by barium sulfate. Because of this irreversible binding the anticoagulant activity of aspirin lasts far longer than its anti-inflammatory effect.
It is an oily colorless liquid with an unpleasant odor. Elimination of mesalazine is mainly via the renal route following metabolism to N-acetyl-5-aminosalicylic acid acetylation. Isobutyric acid 2-methylpropanoic acid is an isomer.
Such compounds are termed acetate esters or simply acetates. The OCOCH 3 group introduced by Dr Felix Hoffmann into the natural moiety of salicylic acid is responsible for turning a weak reversible inhibitor into a potent irreversible inhibitor of the enzyme prostaglandin PGGH-synthase-1 also referred to as cyclooxygenase. The usual therapeutic range of serum salicylate concentration is 1030 mgdl 0722 mmolL.
It introduces an acetyl group into a chemical compound. Obtained by Green Fractionation from the natural essential oil of the Wintergreen plant Neosalyl brings all its benefits in dermo-purification for skin and shininess and anti. 171 172 A major source of these ROS is NADPH oxidase a plasma-membrane-bound enzyme complex from the NOX.
The risk or severity of hypertension can be increased when Mesalazine is combined with Salmeterol. In addition aspirin may irreversibly bind to COX-1 through acetylation of a serine residue near the enzyme active site.

Acetylation Explain Acetylation Reaction Of Acetylation With Mechanism Example And Faqs

Acetylation Explain Acetylation Reaction Of Acetylation With Mechanism Example And Faqs
Chemistry 104 Synthesis Of Aspirin

Synthesis Of Acetyl Salicylic Acid From Salicylic Acid And Acetic Download Scientific Diagram